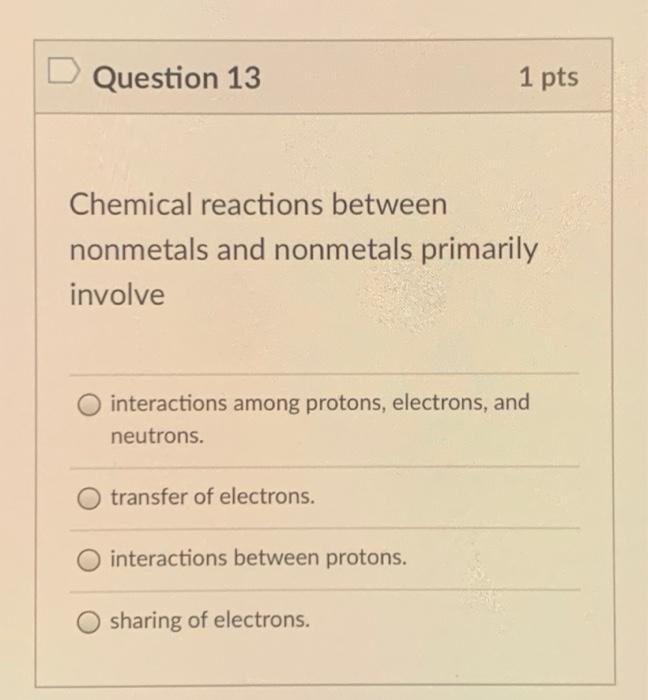
Chemical Reactions Between Nonmetals and Nonmetals Primarily Involve
The sharing of electrons via covalent bonds. How many electrons docs a stable barium ION have.

Solved 1 An Ion Is Formed A By Either Adding Electrons To Chegg Com
Most of these oxides are acidic that is they react with water to form oxyacids.

. Chemical reactions between metals and nonmetals. If you continue browsing the site you agree to the use of cookies on this website. Chemical reactions between metals and nonmetals primarily involve.
D interactions between protons and electrons. Chemical reactions between nonmetals and nonmetals primarily involve a. Interactions between protons and electrons.
This video is about Chemical Properties of Metals and Non-. The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. Their properties and behavior are quite different from those of metals on the left side.
There are five general aspects of the oxidation-reduction. With the exception of most of the noble gases all nonmetals form compounds with oxygen yielding covalent oxides. Non-metals like carbon sulphur and phosphorus react with oxygen to form non-metallic oxides.
The reaction of chlorine with bases like sodium hydroxide gives products like sodium hypochlorite sodium chloride as well as water. Chemical reactions between metals and nonmetals primarily involve A transfer of electrons. The variety of oxidation states displayed by most of the nonmetals means that many of their chemical reactions involve changes in oxidation state through oxidation-reduction reactions.
Reaction between metals and non-metals SlideShare uses cookies to improve functionality and performance and to provide you with relevant advertising. The oxides of non-metals are acidic or neutral in nature. To Learn the major differences between Metals and Nonmetals.
Difference Between Metals and Nonmetals- The elements present on the extreme right side of the periodic tables are non-metal About 80 of the 105 elements in the periodic table are regarded as metals. In a reaction between sodium and chlorine the electron lost by sodium could be picked up by chlorine. Interactions among protons electrons and neutrons.
Chemical reactions between nonmetals and nonmetals primarily involve a. 4Fes 3O2g 2Fe2O3s 0 0 3 2 4 Fe s 3 O 2 g 2 Fe 2 O 3 s 0 0 3 2. They include the most reactive and least reactive of elements and they form many different ionic and covalent compounds.
But all metals have similar chemistry and so do non-metals. The oxidation state of the metal becomes positive as it undergoes oxidation and that of the nonmetal becomes negative as it undergoes reduction. This section presents an overview of the properties and chemical behaviors of the nonmetals as well as the chemistry of specific elements.
Bromine is a versatile substance primarily. Under normal conditions more than half of the nonmetals are gases one is a liquid and the rest include some of the softest and hardest of solids. What Chemical reactions occur between nonmetals and nonmetals.
Oxides of non-metals are formed when it reacts with oxygen. Nonmetals oxidize most metals. These oxides are also called acidic oxides as they form acids when dissolved in water.
Interactions among protons electrons and neutrons. Carbon burns in air oxygen to form carbon dioxide. Chemical reactions between metals and nonmetals primarily involve All the following species are isoelectronic with Ar EXCEPT How many electrons are shown in the Lewis formula for the perbromate ion.
The reaction between non-metals and bases is a very complex one. Bonding between a metal and a nonmetal is often ionic. Interactions between protons and.
C interactions between protons electrons and neutrons. Asked Aug 24 2019 in Chemistry by Valentin. Bonds between two nonmetals are generally covalent.
B interactions between protons. The nonmetals exhibit a rich variety of chemical behaviors. Understand the definition properties uses of metals and non-metals Visit BYJUS for more content.
Because its nucleus has 17 protons and its K L and M shells have 18 electrons the chlorine atom has a negative charge after gaining one electron. Recall from the acid-base chapter that an oxyacid is an acid consisting of hydrogen oxygen and some other element. Chemical Properties of Non Metals.
Every substance has its own chemistry. The nonmetals are elements located in the upper right portion of the periodic table. Some compounds contain both covalent and ionic bonds.
Ionic compounds tend to form between metals and nonmetals when electrons are transferred from an element with high ionization energy metal to an element with a low electron affinity nonmetal.

Chapter 5 Chemical Reactions 5 3 Chemical

Solved Chemical Reactions Between Metals And Nonmentals Chegg Com
Solved D Question 13 1 Pts Chemical Reactions Between Chegg Com
No comments for "Chemical Reactions Between Nonmetals and Nonmetals Primarily Involve"
Post a Comment